PUBLICATIONS AND NEWS
The association of gold mineralization with volcanic
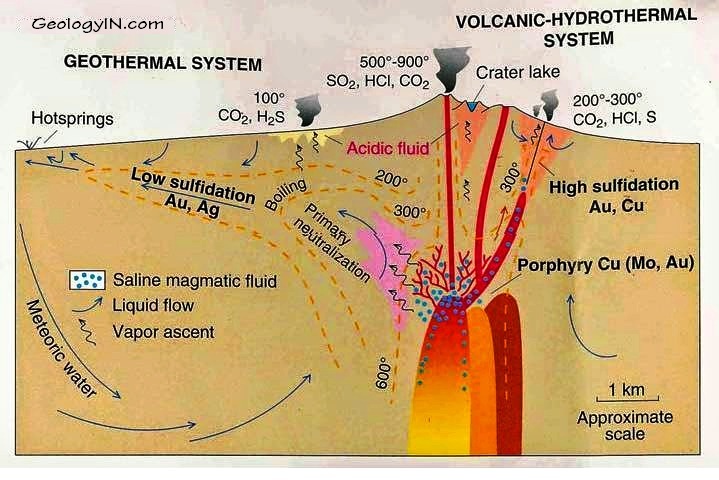
Fluids emanating from a molten magma are extremely hot and under high pressure deep below the surface. As these fluids rise, they mix with surface waters and change the composition of the rocks with which they come into contact. This process is known as alteration. Eventually the fluids breach the surface and form either acidic lakes known as fumaroles common in the craters of volcanoes or dilute, neutral hot springs like those at Yellowstone or the Geysers in California.
These two different surface manifestations – acidic lakes or neutral hot springs – reflect two different fluid types that each result from the two different paths taken by the magma as it rises to the surface. Both form gold deposits and are known respectively as low- and high-sulphidation gold deposits.
In both subtypes gold will largely be precipitated from 2.5 kilometers depth to surface.
Recognizing that gold precipitates near the surface in these systems, the great American geologist Waldemar Lindgren coined the term epithermal in 1933, epi meaning shallow and thermal referring to the heated fluid.
The chemist Werner Giggenbach further subdivided epithermal gold deposits into low and high sulphidation types .
Low and high do not refer to each type’s relative amount of sulphide minerals (metal complexes of sulfur with metals). Rather the distinction is based on the different sulfur to metal ratio within the sulphide minerals of each subtype.
While this discussion deals with high-sulphidation epithermal systems, it is worth mentioning that low-sulphidation systems also form economic gold deposits although they develop under vastly different chemical conditions.